Shikimic Acid 98% Tamiflu Intermediate Pandemic API
Shikimic Acid 98% Tamiflu Intermediate Pandemic API
Shikimic Acid 98% Antiviral API Ingredient
Our Shikimic Acid 98% is a high-purity active pharmaceutical ingredient (API) extracted from Illicium verum (Star Anise) using refined alcohol extraction techniques. With a 98% assay purity, this compound is widely recognized as the essential intermediate for antiviral drugs like Oseltamivir (Tamiflu), making it a strategic component in pandemic preparedness. Compliant with international standards including cGMP, HACCP, Halal, Kosher, SGS, and IAF, our product is ideal for pharmaceutical, nutraceutical, and biochemical manufacturing. Available in bulk with tailored OEM/ODM options, our Shikimic Acid is trusted by industrial buyers across North America, Europe, Asia, and the Middle East.
Description
Shikimic Acid 98%
Shikimic Acid 98% is a naturally occurring cyclohexene compound extracted from Illicium verum, commonly known as star anise. It plays a critical role as a precursor in the synthesis of antiviral medications, particularly Oseltamivir phosphate (Tamiflu), used globally for influenza treatment and pandemic response.
Our product is manufactured using advanced alcohol extraction and purification technologies that ensure exceptional purity and safety. Each batch undergoes strict quality testing and complies with globally recognized certifications including cGMP, HACCP, SGS, Halal, and Kosher.
Whether for pharmaceutical production, chemical synthesis, or R&D applications, our high-purity Shikimic Acid ensures performance, consistency, and compliance across industries.

Shikimic Acid Extraction & Purification Flowchart:
1.Raw Material Sourcing
Sustainably harvested Illicium verum from traceable, non-GMO sources.
2.Initial Cleaning & Drying
Raw botanicals are thoroughly washed and air-dried to eliminate contaminants.
3.Alcohol Extraction
High-grade ethanol extraction is used to isolate shikimic acid compounds while preserving bioactivity.
4.Filtration & Concentration
The extract is filtered and concentrated under controlled conditions to increase shikimic acid content.
5.Purification & Crystallization
Advanced crystallization techniques are applied to achieve 98% purity with consistent assay values.
6.Drying & Milling
The final compound is dried and milled into a fine powder for easy handling and formulation.
7.Testing & Quality Control
Each lot is verified by third-party laboratories for purity, microbial load, heavy metals, and pesticide residues.
8.Packaging
Vacuum-sealed in moisture-resistant, food/pharma-grade packaging with bulk and private label options.

🔹 Certifications
✅ cGMP Certified
✅ HACCP Certified
✅ SGS Third-Party Tested
✅ IAF Accredited
✅ Halal Certified
✅ Kosher Certified
✅ Clean Label
✅ Pesticide-Free & Heavy Metal Tested
🔹 Product Highlights
🧪 High Purity 98%: Ideal for pharmaceutical-grade applications and active compound synthesis.
🌿 Natural Source: Derived from Illicium verum, a renewable botanical source.
🌍 Global Compliance: Meets international quality and safety standards.
📦 Bulk API Supply: Ready for large-scale industrial and pharmaceutical manufacturing.
🧼 Clean & Solvent-Free Residues: Produced using food-grade alcohol extraction only.
🔹 Product Benefits
✅ Essential API Intermediate: Critical raw material for Oseltamivir (Tamiflu), widely used in influenza and pandemic treatments.
✅ Strategic Stockpile Value: Valuable component for governments and healthcare organizations’ pandemic preparedness.
✅ Pharmaceutical-Grade: Consistent assay purity and quality control for regulated markets.
✅ Versatile Use: Applicable in pharma, nutraceuticals, fine chemicals, and R&D labs.
✅ Long Shelf Life: Stable storage in sealed packaging under recommended conditions.

🔹 Application Areas
💊 Pharmaceutical Manufacturing: Core intermediate in antiviral drug synthesis, including Tamiflu.
🧪 Research & Development: Ideal for pharmaceutical R&D and biochemical synthesis processes.
🧬 Biotech & Life Sciences: Suitable for biotech experiments and compound formulation testing.
🏥 Healthcare & Strategic Reserves: Valuable for emergency medical stockpiles and pandemic logistics.
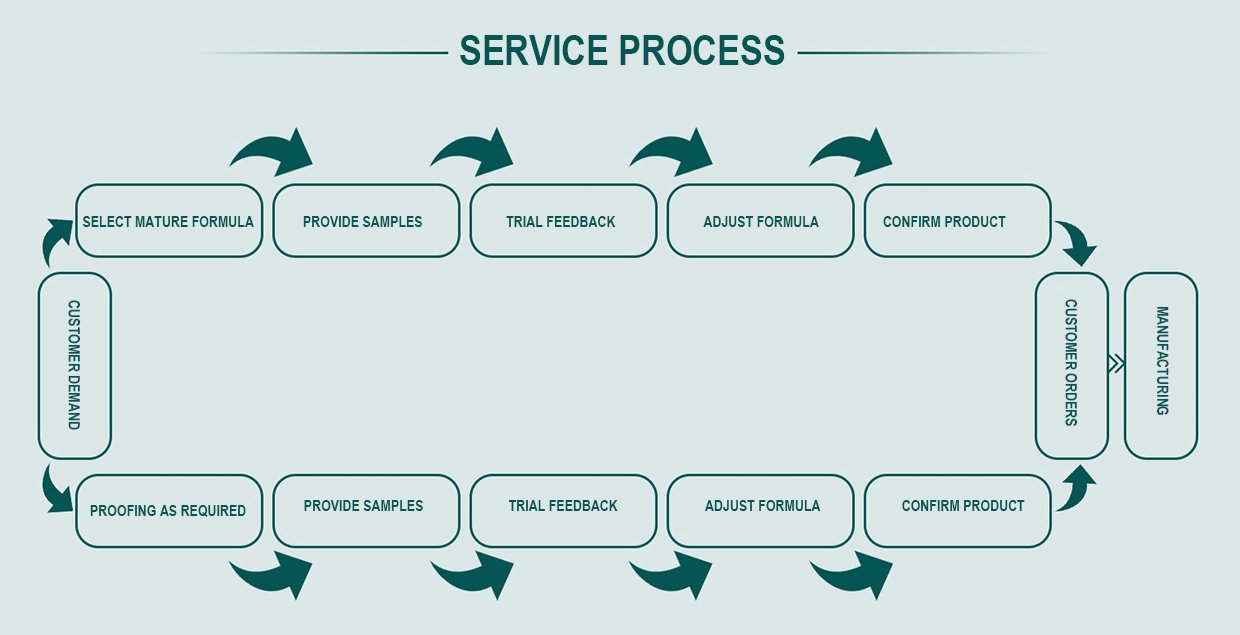
🔹 OEM / ODM Capabilities
🛠️ Custom Specifications: Ability to tailor purity levels, granule size, and packaging formats upon request.
📦 Private Label Packaging: Fully customizable packaging solutions with client branding and regulatory labeling.
🏭 Scalable Production: Capable of small-batch R&D supply or full-scale pharmaceutical-grade bulk production.
🔬 Collaborative R&D: In-house scientific team supports formulation design and clinical application consultation.
Standard plant extract series
| Item No | Product name | Active ingredients | Specification | Item No | Product name | Active ingredients | Specification | |
| 1 | Ginkgo Biloba Extract | Flavonoids,Lactones | 24%,6% | 54 | Mangosteen Extract | AlphaMangostina | 10-90% | |
| 2 | Shitake Mushroom Extract | Polysaccharides (UV) | 30%, 40%,50% | 55 | RoseHipExtract | Flavones | 1-20% | |
| 3 | Ivy Leaf Extract | Total Hederasaponins | 5%-60% | 56 | Schisandra Berry Extract | Schizandrins | 2-9% | |
| 4 | Ginseng Extract | Ginsenosides | 5%-80% | 57 | Cassia Seed Extract | Anthaquinone | 3% | |
| 5 | Ginger Extract | Gingerols | 5%,10%,25% | 58 | Soybean Extract | soy lsoflavones | 40% | |
| 6 | Rhodiola Rosea Extract | Salidroside, Rosavins | 3%-98%,3% | 59 | Chaga Extract Powder | Polysaccharides | 50% | |
| 7 | Ganoderma Extract | Polysaccharides (UV) | 30%,40%,50% | 60 | Hericium Erinaceus Extract | Polysaccharides | 30% | |
| 8 | Red Clover Extract | Total Isoflavones | 8%,10%,20%,40% | 61 | Trametes Versicolor Extract | Polysaccharides | 30% | |
| 9 | Grape Seed Extract | OPC | 95% | 62 | Cordyceps Militaris Extract | Polysaccharides | 30% | |
| 10 | Turmeric Extract | Curcumin | 95% | 63 | Ginkgo Biloba Extract | Ginkgo Flavonoids | 24% | |
| 11 | Quercetin | Quercetin | 95% | 64 | Angelica Sinensis Extract | Ferulic Acid | 1% | |
| 12 | Rutin | Rutin | 95% | 65 | Eleuthero Extract | Eleutherosides | 0.8% | |
| 13 | Diosmin | Diosmin | 90% | 66 | Gymostemma Extract | Gypenosides | 80% | |
| 14 | Hespridin | Hespridin | 90% | 67 | Hawthorn Extract | Proanihocyanidins | 20% | |
| 15 | Marigold Extract | Lutein / Zeaxanthin | 2%-80% | 68 | Ephedra Extract | Ephedrine | 8% | |
| 16 | Griffonia Simplicifolia Extract | 5-HTP | 10%-99% | 69 | Olive Leaf Extract | Oleuropein | 20% | |
| 17 | Kava Extract | Kavalactones | 30%,50%,70% | 70 | Centella Asiatica Extract | Asiaticoside | 40% | |
| 18 | Hawthorn Extract | Flavonoids | 2%,50% | 71 | Calendula Extract | Lutein | 5% | |
| 19 | Hibiscus Flower Extract | Anthocyanins | 5%-25% | 72 | Chamomile Extract | Aigenin | 1% | |
| 20 | Lion’s Mane Mushroom Extract | Polysaccharides (UV) | 30%,40%,50% | 73 | Senna Leaf Extract | Sennosides | 20% | |
| 21 | Maitake Mushroom Extract | Total Sugars (UV) | 30%,40%,50% | 74 | Chicory Extract | lnulin | 20% | |
| 22 | Mangosteen Extract | α-Mangostin | 10%-98% | 75 | Panax Notoginseng Extract | Notognsenosides | 5% | |
| 23 | Apple Cider Vinegar Powder | Apple Cider Vinegar Powder | 5%-50% | 76 | Polygonum Cuspidatum Extract | Resvetrol | 10-98% | |
| 24 | Yucca Extract | Saponins | 10%~98% | 77 | Kudzu root extract | puerarin | 15-98% | |
| 25 | Broccoli Extract | Glucoraphanin | 1%-20% | 78 | Scutellaria Extract | Baicalin | 85% | |
| 26 | Semen Ziziphi Spinosae Extract | Saponins | 1%-10% | 79 | Mulberry Leaf Extract | Sodium copper chlorophylin | 99% | |
| 27 | Echinacea Purpurea Extract | Chicoric Acid | 1%-4% | 80 | Alpha Lipoic Acid | Alpha Lipoic Acid | 99% | |
| 28 | Eucommia Leaf Extract | Chlorogenic Acid | 5%-98% | 81 | Coenzyme Q10 | Coenzyme Q10 | 98% | |
| 29 | Senna Leaf Extract | Sennosides A+B | 6%,8%,10%,20%,30% | 82 | pymoloquinoine Qunone Diodum(sait pqq) | pymoloquinoine Qunone Diodum(sait pqq) | 99% | |
| 30 | Scutellaria Root Extract | Baicalin | 88% | 83 | B-Nicinamide mononucleotie(NMN) | B-Nicinamide mononucleotie(NMN) | 99% | |
| 31 | Bitter Apricot Extract | Amygdalin | 98% | 84 | Astragalus Extract | Astragalus polysaccharides | 5-98% | |
| 32 | Soapnut Extract | Sapindus Saponins | 30%-80% | 85 | Scutellaria Extract | Scutellaria polysaccharide | 30%,50%,70% | |
| 33 | Pine Bark Extract | OPC | 95% | 86 | Green Tea Extract | Green Tea polyphenols | 50-98% | |
| 34 | Berbcrine Hydrochloride | Berberine Hydrochloride | 97% | 87 | GRAPE SKIN EXTRACT | Resveratrol | 5-20% | |
| 35 | Black Garlic Extract | Allicin | 1%-5% | 88 | Grape Seed Extiact | Proanthocyanidins | 95% | |
| 36 | Centella Asiatica Extract | Asiaticoside | 10%-70% | 89 | Turmeric Extract | Curcumin | 95% | |
| 37 | Aloe Yera GelFreeze Dried Powder | Freeze-Dried Aloe Vera Gel Powder | 50:1,100:1,200:1 | 90 | PanaxGinsengExtract | Ginsenosides | 10-80% | |
| 38 | Magnolia Bark Extract | Honokiol | 50%-98% | 91 | Pomegranate Extiact | Ellagic ACid | 40-90% | |
| 39 | Cyanotis Arachnoidea Extract | Ecdysterone | 2%-90% | 92 | Elderberry Extract | Anthocyanidin | 1-25% | |
| 40 | Epimedium Extract | Icariin | 1%-98% | 93 | Passiflora incarnate Extract | Flavone | 5% | |
| 41 | Cactus Extract | Polysaccharides | 10%-30% | 94 | Shiitake Mushroom Extract | Shiitake mushrooms | 10-50% | |
| 42 | Chaga Mushroom Extract | Polysaccharides (UV) | 30%, 40%,50% | 95 | Ganoderma lucidum Extract | Ganoderma lucidum | 10-50% | |
| 43 | Cistanche Desertcola Extract | Total Glycosides | 20%-80% | 96 | white willow Bark extract | Salicin | 15-98% | |
| 44 | Green Tea Extract | EGCG | 30%-98% | 97 | Rhodiola Rosea Extract | Salidroside | 1-3% | |
| 45 | Apple Exttact | Polyphenols | 1-70% | 98 | Marigold Flower Extract | Lutein | 5-20% | |
| 46 | Bilbery Leat Extract | Flavones | 1-20% | 99 | Milk Thistle Extract | Sillymarin | 80% | |
| 47 | Cinnamon Bark Extract | Flavones | 1-30% | 100 | wild Yam Extract | Diosgenin | 20-98% | |
| 48 | Pine Bark Extract Powder | Proanthocyuanidins | 95% | 101 | Echinacea purpurea Extract | Chicory Acid | 1-3% | |
| 49 | Epimedium Extract | ICcarins | 1-98% | 102 | Sophora Japonica Extract | Rutin | 95% | |
| 50 | Grapeseed Extract | CProarthocyanidins | 95% | 103 | Sophora Japonica Extract | Quercetin | 95% | |
| 51 | Goji Extract | Povsaccharides | 5-60% | 104 | Aloe vera Extract | Aloin | 20% | |
| 52 | Rosemary Extract | RosemaryAcid | 5% | 105 | Haematococcus pluvialis Extract | Astaxanthin(Powder/oil) | 5% | |
| 53 | Horsetail Extract | silica | 7% | 106 | Tomato Extract | LYCOPENE(Powder/oil) | 5%,10% |
Frequently Asked Questions (FAQ) – Shikimic Acid 98%
1. What is Shikimic Acid 98%?
Shikimic Acid 98% is a high-purity active pharmaceutical ingredient (API) extracted from Illicium verum (Star Anise) using refined alcohol extraction. It has ≥98% purity and is widely used as an essential intermediate for antiviral drugs like Oseltamivir (Tamiflu).
2. What are the main applications of Shikimic Acid?
Shikimic Acid is commonly used in:
-
Pharmaceutical manufacturing (especially antiviral drugs)
-
Nutraceuticals and functional health products
-
Biochemical and chemical research
-
Industrial production requiring high-purity intermediates
3. How is your Shikimic Acid produced?
Our Shikimic Acid is extracted from star anise using advanced alcohol-based extraction techniques and purified to ensure consistent ≥98% assay purity.
4. What certifications does your Shikimic Acid carry?
It complies with cGMP, HACCP, SGS, IAF, Halal, and Kosher standards, making it suitable for global regulatory markets.
5. Is Shikimic Acid suitable for pharmaceutical and nutraceutical applications?
Yes. Its high purity and clean-label profile make it ideal for pharmaceutical intermediates, research-grade compounds, and nutraceutical formulations, depending on local regulations.
6. Do you offer OEM/ODM services?
Yes. We provide custom OEM/ODM solutions, including bulk packaging, specific purity requirements, and formulation support for global industrial buyers.
7. What forms are available?
Shikimic Acid 98% is supplied as a fine crystalline powder. Bulk quantities and customized packaging are available for industrial-scale production.
8. How should Shikimic Acid be stored?
Store in a cool, dry place, protected from light and moisture. Keep the container tightly sealed to maintain stability and shelf life.
9. Can samples be requested before bulk purchase?
Yes. Samples are available for quality testing, research, and R&D purposes before placing bulk orders.
10. What is the typical lead time for orders?
Standard bulk orders usually ship within 3–7 business days, while customized OEM/ODM solutions may require additional production time.





Reviews
There are no reviews yet.